NanoBiomolecular Group
Istituto di Scienze Applicate e Sistemi Intelligenti “E.Caianiello”
CONSIGLIO NAZIONALE DELLE RICERCHE


NanoBiomolecular Group
Istituto di Scienze Applicate e Sistemi Intelligenti “E.Caianiello”
CONSIGLIO NAZIONALE DELLE RICERCHE


NanoBiomolecular Group
Istituto di Scienze Applicate e Sistemi Intelligenti “E.Caianiello”
CONSIGLIO NAZIONALE DELLE RICERCHE

HYHEAT
Profiling gene expression in Hydra vulgaris following
Gold Nanoparticle-mediated hyperthermia

MCSA- IF-2014
Hyperthermia (HT) is currently used as a non-invasive technique for cancer therapy, whereby biological tissues are exposed to higher than normal temperatures, for selective ablation of tumoral cells. Heating treatments can be applied using external heating sources such as ultrasounds, however,heating only malignant cells selectively is difficult to obtain, and therefore the translation of this modality to the clinic is still challenging. Recently, there has been a growing interest in the use of gold nanoparticles (AuNPs) to selectively generate heat in a spatiotemporal fashion, which is known as photothermal therapy (PTT). The rationale for such therapy is based on the fact that metallic NPs can be synthetized to absorb incident light coming from a laser outside the body and generate heat only in the tissues where NPs are allocated. Moreover, AuNPs are highly biocompatible and can be finely tuned to absorb light in the near-infrared (NIR) spectral range, known as the “biologicalwindow” (690-1100 nm). In this range tissue is maximally transparent, and therefore, the light itself
would not damage the tissue.
While significant efforts have been made to develop suitable NPs with appropriate heating capabilities, to date, the molecular mechanisms underlying the in vivo cellular responses to heat stress remain unclear.
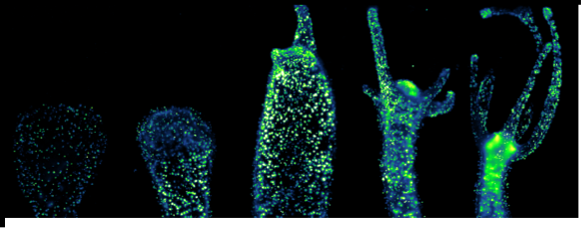
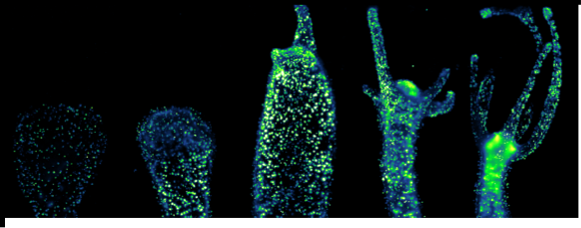
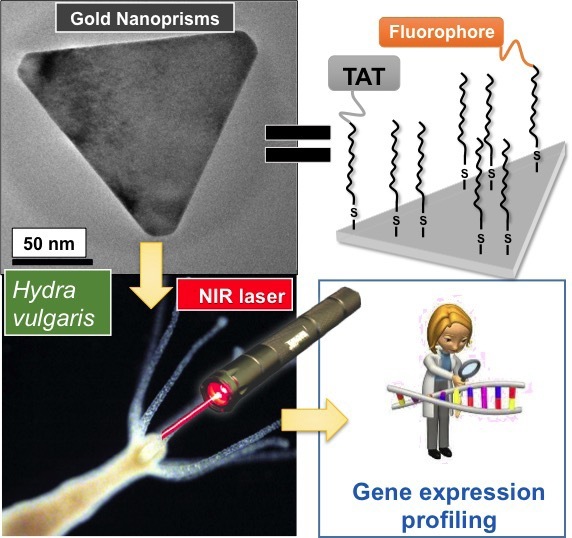
Therefore, the overall aim of the HyHeat project is to use an invertebrate model (Hydra vulgaris) to screen the heating capabilities of different AuNPs in vivo, with the grand aim of understanding the cellular responses to heat stress and therefore taking the first steps towards improving nanoparticle mediated HT efficacy for therapeutic purposes. A simple invertebrate organism have been used, in line with European strategies aimed to reduce vertebrate experimentation. During the project, we have synthesized different types of AuNPs and fully characterized them. The toxicity in the animals have been assessed using different techniques, before proceeding to laser irradiation. Lastly, gene expression profiling after laser irradiation in Hydra has been performed. It has been possible to characterize the heating capabilities of the AuNPs in vivo and select deregulated genes upon irradiation.
RESULTS: The objectives proposed in the project have been successfully accomplished . By determining the gene expression profile after laser irradiation, it has been shown which AuNPs perform as better nanoheaters in vivo. This can help to take the first steps towards improving PTT efficacy for clinical purposes. Also, the main genes deregulated after irradiating the animals have been assessed, both in mild and sublethal conditions. In conclusion, Hydra may serve as an excellent model to reduce vertebrate experimentation, which is in line with guidelines of ECVAM for the development of new testing species.
Publication:
Moros M, Lewinska A, Merola F, Ferraro P, Wnuk M, Tino A, Tortiglione C. Gold Nanorods and Nanoprisms Mediate Different Photothermal Cell Death Mechanisms In Vitro and In Vivo. ACS Appl Mater Interfaces. 2020 Mar 25;12(12):13718-13730

Go to: HYPERTHERMIA
Go to Hyperthermia